
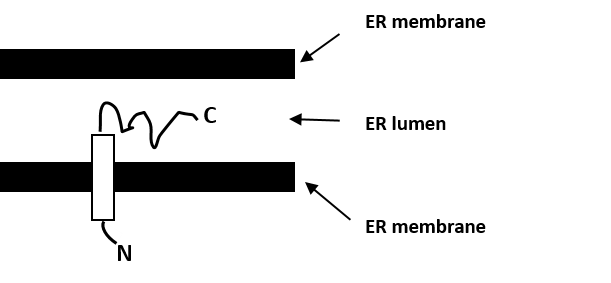
In contrast, the fluorescence in the GFPHO1 ( Figure 1C) and in the HO1-GFP ( data not shown) expressing cells was not affected by permeabilization. Cells transfected with an unconjugated GFP construct, which is a soluble cytosolic protein, lost most of their fluorescence within 60 s of digitonin permeabilization ( data not shown). In addition, we demonstrated that the constructs were membrane-bound just like endogenous HO1 by permeabilizing transfected cells with digitonin. Also, the cellular distribution of the constructs appeared identical to endogenous HO1 ( Figure 1B). To determine if the GFP constructs are localized to the ER-like endogenous HO1, the fusion constructs were transfected to J774.1 cells and co-localized with the ER protein Calnexin ( Figure 1B). To study the orientation of HO1 in the ER membrane, three fluorescent HO1 constructs, GFP-HO1, HO1-GFP and CFP-HO1-GFP were engineered ( Figure 1A and E). The cytosolic orientation of HO1 was determined with this method and confirmed by Western blot. Fluorescence was protected in constructs where the fluorescent protein faced the lumen of a subcellular compartment. Addition of protease-digested membrane-bound proteins abolished the fluorescence of the construct when the fluorescent protein was facing the cytosol. The plasma membranes of cells expressing various conjugates of HO1 with fluorescent proteins were selectively permeabilized by digitonin in vivo and cytosolic soluble proteins leaked from these cells. The fluorescence protease protection assay (FPP) 11 and Western blotting were used to determine the subcellular localization and orientation of HO1 in vivo. 10 However, the orientation of HO has never been studied in intact cells. 10 Studying microsomal rat liver fractions, it was suggested that the orientation of HO is not towards the microsomal lumen, as the major soluble (N-terminal) part was cleavable by trypsin from the microsomal fraction in vitro. HOs have no ER targeting sequence, and membrane anchoring can occur spontaneously into microsomal membranes but not into RBC membranes. The HOs have a single C-terminal transmembrane region 8 and are considered ER-anchored 9 due to their enrichment in microsomal fractions.
#CYTOSOL AND ER LUMEN FULL#
But little is known about the chaperones that can transport hydrophobic heme through the aqueous cytosol, and a full pathway for heme transport from the phagosome to the endoplasmic reticulum (ER)-bound HO1 still needs to be clarified.

6 HRG-1 is expressed in the endo-lysosomal system 7 and is, therefore, a good candidate to transport heme released from hemoglobin to the cytosolic compartment. In recent years, several heme transporters have been cloned, including the heme importer HRG-1. 5 The fact that most heme targeted for breakdown is released in membrane-bound compartments implies that heme needs to be transported to the site of its degradation or the enzyme needs to be recruited to the site of heme release. 4 Approximately 85% of all heme degraded in mammals comes from hemoglobin. HO1 and HO2 break down heme into iron, carbon monoxide (CO) and biliverdin, and this pathway is the main physiological heme degradation system. Nitric oxide synthase is digested by the proteasome and therefore its heme is released to the cytosol 2 while other heme proteins, such as hemoglobin, catalase and all the mitochondrial heme proteins release their heme into membranebound vesicles of the phago-endo-lysosomal system. The subcellular location of heme protein degradation is not uniform. 1 When released from hemoproteins, heme is degraded by heme-oxygenase (HO)1 or HO2. Heme is a highly hydrophobic molecule embedded in heme proteins and is used as a carrier and sensor of gases, as an electron donor and acceptor, and as a catalyst in many enzymatic reactions.


 0 kommentar(er)
0 kommentar(er)
